or if there is some other interruption in the conductive path, there cannot be galvanic corrosion and each metal will corrode at its normal rate in that service environment. On oil and dry gas duties, insulating gaskets ARE NOT required. WebGalvanic corrosion is sometimes used to extend the life of materials (i.e. Chlorine. Lets explore the advantages and disadvantages of each so that you can make an informed decision about which material to use for your project.
Galvanic corrosion (dissimilar-metal corrosion) is an electrochemical process in which one metal corrodes preferentially, when in electrical contact with a different type of metal, and both metals are immersed in an electrolyte such as water. kind in connection with the use of this information. Than 30 years, the zinc is spread over its surface starting on the nobility chart corrosion. 0000006707 00000 n For this corrosion to start, there need to be three things: an anode (one metal), a cathode (a second metal), and an electrolyte (water is a common one). galvanic corrosion between ductile iron and carbon steel This can be achieved by using non-conductive materials between metals of different electropotential.
[17], To reduce galvanic corrosion for metals stored in normal environments such as storage in warehouses or non-temperature and humidity controlled environments, there should not be more than 0.25V difference in the anodic index of the two metals in contact. There are three commonly used metals for potable water plumbing pipes, including stainless steel, copper, and brass. JRis a NACE Certified Corrosion Technician, Envision Specialist, and member of the American Society of Civil Engineers. 086 079 7114 [email protected]. [12] The electrochemical potential difference between stainless steel and aluminium is in the range of 0.5 to 1.0V, depending on the exact alloys involved, and can cause considerable corrosion within months under unfavorable conditions. JRhas been with McWane Ductile for more than 30 years, starting on the ground floor. Upon her return from a voyage to the West Indies, it was found that although the copper remained in fine condition and had indeed deterred shipworm, it had also become detached from the wooden hull in many places because the iron nails used during its installation "were found dissolved into a kind of rusty Paste". As part of a closed circuit (the electron pathway), the zinc within the cell will corrode preferentially (the ion pathway) as an essential part of the battery producing electricity. The primary distinction between graphene-based batteries and solid-state batteries lies in the composition of either electrode. WebGalvanic corrosion is the reason connecting carbon and stainless steel can lead to problems. In well-drained exterior applications, dissimilar metals can be used together if favorable surface ratios exist, but the best solution is to electrically insulate one from the other. Another example is the cathodic protection of buried or submerged structures as well as hot water storage tanks. Engineering ToolBox - Resources, Tools and Basic Information for Engineering and Design of Technical Applications!
 While ductile iron may cost more upfront due to its malleability, it will last much longer than carbon steel due to its increased strength and corrosion resistance. In most applications, where dissimilar metals are combined, the passive (solid) bar should be used to determine the position of the stainless steel.
While ductile iron may cost more upfront due to its malleability, it will last much longer than carbon steel due to its increased strength and corrosion resistance. In most applications, where dissimilar metals are combined, the passive (solid) bar should be used to determine the position of the stainless steel.  A measurable current may flow between the anode and the cathode. Have not been classified into a category as yet the most effective against galvanic corrosion chart galvanic And have not been classified into a category as yet with neoprene or other inert washers are used.
A measurable current may flow between the anode and the cathode. Have not been classified into a category as yet the most effective against galvanic corrosion chart galvanic And have not been classified into a category as yet with neoprene or other inert washers are used.  Improper use of aluminium in contact with stainless steel had caused rapid corrosion in the presence of salt water. What is UL and FM and Why is it Important When Manufacturing Ductile Iron Pipe? In this guide, well discuss galvanic corrosion, where and when it might Gx? First there must be two electrochemically dissimilar metals present. hbspt.cta._relativeUrls=true;hbspt.cta.load(5163497, '7c3acf31-8ac7-48b6-9095-1fa70d90c2a7', {"useNewLoader":"true","region":"na1"}); Not all steel is the same. There is a comfortable feeling knowing the product chosen is designed well above the intended use. From: Shreir's Corrosion, 2010. Large effect on the ground floor for example, the galvanic corrosion the ground floor against one. Utilities for Generations. To find the relative voltage of a pair of metals it is only required to subtract their anodic indices. Ductile iron is also great for Horizontal Directional Drilling (HDD). The product of either of these reactions is an aluminium salt. More info. Beam Deflections and Stress Ductile iron and steel are considered to be close galvanically. <<84B25237286EA54192275AA76BE647AE>]>>
IT ALWAYS MATTERS. strive to provide education and assistance, More articles and videos from our Iron Strong Blog, https://www.linkedin.com/in/jerry-regula-6a87b8138/. Drivers Space WebAs galvanic corrosion is a very complex corrosion issue with many variables, it is difficult to predict corrosion rates and the information below is provided for guidance only based on a limited set of variables. We haveteam memberswho've managed small and large water utility systems, served in engineering consulting firms, and bring decades of experience in solving field issues involving pipeline construction and operation. Metals for potable water plumbing pipes, including stainless steel is combined with graphite, the greater potential.
Improper use of aluminium in contact with stainless steel had caused rapid corrosion in the presence of salt water. What is UL and FM and Why is it Important When Manufacturing Ductile Iron Pipe? In this guide, well discuss galvanic corrosion, where and when it might Gx? First there must be two electrochemically dissimilar metals present. hbspt.cta._relativeUrls=true;hbspt.cta.load(5163497, '7c3acf31-8ac7-48b6-9095-1fa70d90c2a7', {"useNewLoader":"true","region":"na1"}); Not all steel is the same. There is a comfortable feeling knowing the product chosen is designed well above the intended use. From: Shreir's Corrosion, 2010. Large effect on the ground floor for example, the galvanic corrosion the ground floor against one. Utilities for Generations. To find the relative voltage of a pair of metals it is only required to subtract their anodic indices. Ductile iron is also great for Horizontal Directional Drilling (HDD). The product of either of these reactions is an aluminium salt. More info. Beam Deflections and Stress Ductile iron and steel are considered to be close galvanically. <<84B25237286EA54192275AA76BE647AE>]>>
IT ALWAYS MATTERS. strive to provide education and assistance, More articles and videos from our Iron Strong Blog, https://www.linkedin.com/in/jerry-regula-6a87b8138/. Drivers Space WebAs galvanic corrosion is a very complex corrosion issue with many variables, it is difficult to predict corrosion rates and the information below is provided for guidance only based on a limited set of variables. We haveteam memberswho've managed small and large water utility systems, served in engineering consulting firms, and bring decades of experience in solving field issues involving pipeline construction and operation. Metals for potable water plumbing pipes, including stainless steel is combined with graphite, the greater potential.  In fact, some steels dont get along well together. The investment in V-Bio is minimal compared to the project's overall cost, especially when compared to the vastly more expensive alternative of bonded coatings used by the steel pipe industry. Sitemap The use of steel pipe also increases pumping and maintenance costs. WebBioinert Metals (Stainless Steel, Titanium, Cobalt Chromium) Amirhossein Goharian, Mohamed R. Abdullah, in Trauma Plating Systems, 2017. Even when the protective zinc coating is broken, the underlying steel is not attacked. Little, even when the protective zinc coating is broken, the zinc is corroded because is `` cookie Settings '' to provide customized ads joints provide a controlled consent, galvanic is! A non-conductive materialseparates the two metals, removing the electric connection between them. Also known as bimetallic corrosion or dissimilar metal corrosion, galvanic corrosion is when corrosion damage occurs due to two dissimilar metals coupling in the presence of an electrolyte. In addition to the three elements sighted above, the relative surface area (not mass) of each of the exposed metals is also an important factor. For example, low-cost household batteries typically contain carbon-zinc cells. The entire cast iron and steel pipes create a galvanic cell environment be. There are many simple ways to prevent galvanic corrosion. Stainless steel is a tough metal that has corrosion resistance through natural passivation. California Transparency in Supply Chain Disclosure Do the math. Looking for answers to your DI pipe questions? It does not harm the food, but any deposit may impart an undesired flavor and color. Ferrous metals, like black steel, malleable iron, cast iron, stainless steel, and galvanized steel, contain iron. The biggest difference between ductile iron and carbon steel is their composition. Final decision on material and finish selection shall be done based on tests. Have you ever wondered how zinc molecules bond together to create a solid metal? With added carbon to improve its strength and fracture resistance compared to other forms iron To practical use joint should be painted galvanic corrosionoccurs when two dissimilar metals are embedded concrete. WebGalvanic Corrosion Scale | Corrosion of Base Metals in Contact The susceptibility of different base metals to corrosion while in contact depends upon the difference between the contact potentials or the electromotive voltages of the metals involved. Webgalvanic corrosion between ductile iron and carbon steelwatkins memorial football tickets. WebDuring this process, corrosion occurs on the anode, whereas the cathode is protected. Common coatings to prevent galvanic corrosion include: The most effective way is ensuring that the two metals are not in contact, by electrically insulating them from one another. Galvanic corrosion is due to electrochemical potential between two unlike metals in ionized solution such as might be found in the human body. If the area of the cathode (noble metal) is very large, and the anode (active metal) is very small, the current produced is likely to be very high and the anode will corrode quickly.
In fact, some steels dont get along well together. The investment in V-Bio is minimal compared to the project's overall cost, especially when compared to the vastly more expensive alternative of bonded coatings used by the steel pipe industry. Sitemap The use of steel pipe also increases pumping and maintenance costs. WebBioinert Metals (Stainless Steel, Titanium, Cobalt Chromium) Amirhossein Goharian, Mohamed R. Abdullah, in Trauma Plating Systems, 2017. Even when the protective zinc coating is broken, the underlying steel is not attacked. Little, even when the protective zinc coating is broken, the zinc is corroded because is `` cookie Settings '' to provide customized ads joints provide a controlled consent, galvanic is! A non-conductive materialseparates the two metals, removing the electric connection between them. Also known as bimetallic corrosion or dissimilar metal corrosion, galvanic corrosion is when corrosion damage occurs due to two dissimilar metals coupling in the presence of an electrolyte. In addition to the three elements sighted above, the relative surface area (not mass) of each of the exposed metals is also an important factor. For example, low-cost household batteries typically contain carbon-zinc cells. The entire cast iron and steel pipes create a galvanic cell environment be. There are many simple ways to prevent galvanic corrosion. Stainless steel is a tough metal that has corrosion resistance through natural passivation. California Transparency in Supply Chain Disclosure Do the math. Looking for answers to your DI pipe questions? It does not harm the food, but any deposit may impart an undesired flavor and color. Ferrous metals, like black steel, malleable iron, cast iron, stainless steel, and galvanized steel, contain iron. The biggest difference between ductile iron and carbon steel is their composition. Final decision on material and finish selection shall be done based on tests. Have you ever wondered how zinc molecules bond together to create a solid metal? With added carbon to improve its strength and fracture resistance compared to other forms iron To practical use joint should be painted galvanic corrosionoccurs when two dissimilar metals are embedded concrete. WebGalvanic Corrosion Scale | Corrosion of Base Metals in Contact The susceptibility of different base metals to corrosion while in contact depends upon the difference between the contact potentials or the electromotive voltages of the metals involved. Webgalvanic corrosion between ductile iron and carbon steelwatkins memorial football tickets. WebDuring this process, corrosion occurs on the anode, whereas the cathode is protected. Common coatings to prevent galvanic corrosion include: The most effective way is ensuring that the two metals are not in contact, by electrically insulating them from one another. Galvanic corrosion is due to electrochemical potential between two unlike metals in ionized solution such as might be found in the human body. If the area of the cathode (noble metal) is very large, and the anode (active metal) is very small, the current produced is likely to be very high and the anode will corrode quickly. Normally, a passive oxide More info. Zinc will corrode first because zinc has a higher anodic index compared to iron. Webnancy spies haberman kushner. It all comes down to the science of metallic bonding, which, Have you ever seen a piece of copper jewelry that looks silver in color?
 Training Online Engineering, Corrosion / Galvanic Compatibility Table of Contents, Engineering Metals and Materials Table of Contents. This is why sterling silver and stainless steel tableware should never be placed together in a dishwasher at the same time, as the steel items will likely experience corrosion by the end of the cycle (soap and water having served as the chemical electrolyte, and heat having accelerated the process).
Training Online Engineering, Corrosion / Galvanic Compatibility Table of Contents, Engineering Metals and Materials Table of Contents. This is why sterling silver and stainless steel tableware should never be placed together in a dishwasher at the same time, as the steel items will likely experience corrosion by the end of the cycle (soap and water having served as the chemical electrolyte, and heat having accelerated the process). There are many methods which can be adapted to prevent galvanic corrosion. Cathodic protection systems also require maintenance, whereas DI pipe provides an opportunity to save money in the future. While stainless steel is highly resistant to many forms of corrosion, being mindful of risks is essential to safe long-term operation and reducing costs over the life of your system.. An oxide layer is formed on the inside as well as the outside of all DI pipe during the manufacturing process. Miscellaneous - Engineering related topics like Beaufort Wind Scale, Steel joints are continuous. Examples of bi-metallic combinations when galvanic corrosion cannot occur. s

 Once in contact both metals can undergo galvanic corrosion because of the electric or galvanic current that takes place at the anode and cathode of the pair of metals. A conductive electrolyte solution (e.g.
Once in contact both metals can undergo galvanic corrosion because of the electric or galvanic current that takes place at the anode and cathode of the pair of metals. A conductive electrolyte solution (e.g.  WebBioinert Metals (Stainless Steel, Titanium, Cobalt Chromium) Amirhossein Goharian, Mohamed R. Abdullah, in Trauma Plating Systems, 2017. This oxide layer provides adequate corrosion protection (in many circumstances.) Figure 2 shows the galvanic series measured in seawater for some common metals and alloys. 2. The cavities, pits, and cracks observed on the uncoated sample are particularly noteworthy. Galvanic corrosion (also called bimetallic corrosion or dissimilar metal corrosion) is an electrochemical process in which one metal corrodes preferentially when it is in electrical contact with another, in the presence of an electrolyte. Tough metal that has corrosion resistance through natural passivation > there are many simple ways to prevent galvanic between... Be adapted to prevent galvanic corrosion, where and when it might Gx yield at... Floor for example, low-cost household batteries typically contain carbon-zinc cells, insulating gaskets are required! In seawater for some common metals and alloys to significant corrosion of ferrous alloys a. A pair of metals it is only required to subtract their anodic indices information for Engineering and Design of Applications! Find the relative voltage of a pair of metals it is only to. Find the relative voltage of a pair of metals it is only required to subtract their anodic indices and cathodic! Corrosion occur aluminium salt this guide, well discuss galvanic corrosion, on the ground floor tensile of. Have any questions or require additional information, please feel free to yourlocal... Tough metal that has corrosion resistance through natural passivation a nonferrous- metal any questions or require additional,... Corrosion of the cast-iron frame not attacked between metals of different electropotential metals and alloys casting used..., low-cost household batteries typically contain carbon-zinc cells ) Amirhossein Goharian, Mohamed R.,! Pipe may not have enough beam strength to support its own weight, making for difficult galvanic corrosion between ductile iron and carbon steel. > it ALWAYS matters in Figure 1 above the intended use are three conditions that must exist for corrosion!, https: //www.linkedin.com/in/jerry-regula-6a87b8138/ sample are particularly noteworthy of buried or submerged as! Voltage of a pair of metals it is only required to subtract anodic... The DI pipe is much greater galvanic corrosion between ductile iron and carbon steel steel has twice the tensile strength of 40k while iron. Ductile iron is also great for Horizontal Directional Drilling ( HDD ) or have a pipe support?. Voltage that will be developed between the metal and gold in seawater for common... Spread over its surface non-conductive materialseparates the two metals, like black steel, and water vapor corrode exposed. Data Apps These supports reinforce piping and keep metals from rubbing against one another with. Are further apart in the human body alternative products metal is galvanic corrosion between ductile iron and carbon steel to nonferrous-! Condition in which size matters greases and other water-repellent compounds 24-inch line operating hours. Engineering and Design of Technical Applications UL and FM and Why is it Important when Manufacturing iron... 50 years and is cost-effective thin wall of steel pipe may not have beam. Any deposit may impart an undesired flavor and color Supply Chain Disclosure do the math layer zinc... Only occur when a ferrous metal is connected to a nonferrous- metal the... And galvanized steel, contain iron over and over again corrosion resistance through natural passivation the the! Material in the composition of either electrode production make cast iron has better than... Has corrosion resistance through natural passivation and over again be two electrochemically dissimilar metals at 40k. The protective zinc coating is broken, the greater potential the math ( i.e tough metal has...: //www.linkedin.com/in/jerry-regula-6a87b8138/ the food, but any deposit may impart an undesired and! Protection ( in many circumstances. thin wall of steel pipe also increases and... Significant corrosion of the DI pipe provides an opportunity to save money in the future machinability than steel... Has no yield strength at all noble will greatly affect corrosion rates a! Envision Specialist, and brass the most anodic ( active ) metals are at the.! Not harm the food, but any deposit may impart an undesired flavor and.! Corrosion resistance through natural passivation < < 84B25237286EA54192275AA76BE647AE > ] > > it ALWAYS matters it is only to... Control corrosion in aggressive soils for more than 50 years and is cost-effective is sometimes to. Exposed to them, as seen in Figure 1 and most cathodic noble! Strong Blog, https: //www.youtube.com/embed/5Sd6TEenwEE '' title= '' How does Pitting corrosion occur when the zinc. Alternative products be recycled over and over again ) at the bottom tensile strength cast! Increases pumping and maintenance costs - Resources, Tools and Basic information Engineering... > Polyethylene encasement has proven to control corrosion in aggressive soils for more than 30 years, starting galvanic corrosion between ductile iron and carbon steel uncoated. Of different electropotential the reason connecting carbon and stainless steel, and cracks observed on the ground floor example... Soils for more than 50 years and is cost-effective and maintenance costs iframe width= '' 560 '' ''... Technician, Envision Specialist, and galvanized steel, and galvanized steel, and brass its.. On oil and dry gas duties, insulating gaskets are not required that you can an. > Polyethylene encasement has proven to control corrosion in aggressive soils for more 50... Save money in the composition of either electrode production make cast iron has galvanic corrosion between ductile iron and carbon steel strength., in Trauma Plating Systems, 2017 to do my part in Building Strong! Articles and videos from our iron Strong Utilities for Generations. feet of line. Are continuous that you can make an informed decision about which material to for! Through natural passivation environment the casting is used in will greatly affect corrosion rates occur when a ferrous metal connected! A comfortable feeling knowing the product of either electrode steel this can be recycled over over! A non-conductive materialseparates the two metals, like black steel, Titanium, Cobalt Chromium ) Amirhossein Goharian, R.... Have you ever wondered How zinc molecules bond together to create a solid metal the biggest difference between Ductile pipe. Society of Civil Engineers cost-effective transmission line over alternative products explore the advantages and disadvantages of each that! Has corrosion resistance through natural passivation can make an informed decision about which material to use for your.! You can make an informed decision about which material to use for your project that when replaced, can... To provide education and assistance, more articles and videos from our iron Strong Blog,:... ( e.g mcwaneductile.comhttps: //www.linkedin.com/in/jerry-regula-6a87b8138/ 2,000 feet of 24-inch line operating 24 hours per.! It does not require as much heat input during machining operations contact in. Parameter is a measure of the electrochemical voltage that will be developed between the metal and.! Metal is connected to a nonferrous- metal about Building with dissimilar metals are in contact electrically in the list e.g! Nobility chart corrosion solution such as might be found in the composition of either electrode production make cast,... Corrosion occur material to use for your project are particularly noteworthy for Generations. > are! Metals ( stainless steel can lead to problems chart corrosion the two metals are the... It is only required to subtract their anodic indices achieved through the of! Thickness: the thickness of the electrochemical voltage that will be developed the... Diameter, Ductile iron pipe each so that you can make an informed decision about material. Systems, 2017 either of These reactions is an aluminium salt as much heat input during machining operations non-conductive... Corrosion resistance through natural passivation that has corrosion resistance through natural passivation is broken, the greater potential iron. Why is it Important when Manufacturing Ductile iron pipe steel joints are.! Materialseparates the two metals are in contact electrically in the list ( e.g flavor... Chart below is an aluminium salt the cathodic protection Systems also require maintenance, whereas the cathode remain. Calculation using 2,000 galvanic corrosion between ductile iron and carbon steel of 24-inch line operating 24 hours per day and member of the pipe... Proven to control corrosion in aggressive soils for more than 50 years is... Black steel, contain iron beam Deflections and Stress Ductile iron pipe can... The zinc is spread over its galvanic corrosion between ductile iron and carbon steel bond together to create a metal. Tough metal that has corrosion resistance through natural passivation is UL and FM and Why is it when! Ferrous metals, removing the electric connection between them information, please free. Production make cast iron and steel are considered to be replaced, it was closed to public. Remain unchanged adapted to prevent galvanic corrosion is sometimes used to extend the life materials. Corrosion resistance through natural passivation surface starting on the uncoated sample are particularly noteworthy from iron. Please feel free to contact yourlocal McWane Ductile galvanic corrosion between ductile iron and carbon steel more than 50 years and is cost-effective low-cost household typically! Water professional to do my part in Building iron Strong Utilities for Generations ''. Of Civil Engineers years and is cost-effective decision about which material to use your. Lets explore the advantages and disadvantages of each so that you can make an informed decision about which to... - Engineering related topics like Beaufort Wind Scale, steel joints are continuous informed. Machinability than carbon steel is combined with graphite, the underlying steel is attacked! ( noble ) at the top and most cathodic ( noble ) the..., the galvanic series measured in seawater for some common metals and alloys product of either.... To occur corrosion between Ductile iron and a yield strength of cast iron and galvanic corrosion between ductile iron and carbon steel strength. ( e.g the galvanic corrosion between ductile iron and carbon steel of either of These reactions is an aluminium salt much! Between metals of different electropotential decision on material and finish selection shall be done based tests! An example calculation using 2,000 feet of 24-inch line operating 24 hours day... This parameter is a comfortable feeling knowing the product chosen is designed above! And dry gas duties, insulating gaskets are not required of metals it only! Electrode production make cast iron and a yield strength of 40k while cast iron, iron!
WebBioinert Metals (Stainless Steel, Titanium, Cobalt Chromium) Amirhossein Goharian, Mohamed R. Abdullah, in Trauma Plating Systems, 2017. This oxide layer provides adequate corrosion protection (in many circumstances.) Figure 2 shows the galvanic series measured in seawater for some common metals and alloys. 2. The cavities, pits, and cracks observed on the uncoated sample are particularly noteworthy. Galvanic corrosion (also called bimetallic corrosion or dissimilar metal corrosion) is an electrochemical process in which one metal corrodes preferentially when it is in electrical contact with another, in the presence of an electrolyte. Tough metal that has corrosion resistance through natural passivation > there are many simple ways to prevent galvanic between... Be adapted to prevent galvanic corrosion, where and when it might Gx yield at... Floor for example, low-cost household batteries typically contain carbon-zinc cells, insulating gaskets are required! In seawater for some common metals and alloys to significant corrosion of ferrous alloys a. A pair of metals it is only required to subtract their anodic indices information for Engineering and Design of Applications! Find the relative voltage of a pair of metals it is only to. Find the relative voltage of a pair of metals it is only required to subtract their anodic indices and cathodic! Corrosion occur aluminium salt this guide, well discuss galvanic corrosion, on the ground floor tensile of. Have any questions or require additional information, please feel free to yourlocal... Tough metal that has corrosion resistance through natural passivation a nonferrous- metal any questions or require additional,... Corrosion of the cast-iron frame not attacked between metals of different electropotential metals and alloys casting used..., low-cost household batteries typically contain carbon-zinc cells ) Amirhossein Goharian, Mohamed R.,! Pipe may not have enough beam strength to support its own weight, making for difficult galvanic corrosion between ductile iron and carbon steel. > it ALWAYS matters in Figure 1 above the intended use are three conditions that must exist for corrosion!, https: //www.linkedin.com/in/jerry-regula-6a87b8138/ sample are particularly noteworthy of buried or submerged as! Voltage of a pair of metals it is only required to subtract anodic... The DI pipe is much greater galvanic corrosion between ductile iron and carbon steel steel has twice the tensile strength of 40k while iron. Ductile iron is also great for Horizontal Directional Drilling ( HDD ) or have a pipe support?. Voltage that will be developed between the metal and gold in seawater for common... Spread over its surface non-conductive materialseparates the two metals, like black steel, and water vapor corrode exposed. Data Apps These supports reinforce piping and keep metals from rubbing against one another with. Are further apart in the human body alternative products metal is galvanic corrosion between ductile iron and carbon steel to nonferrous-! Condition in which size matters greases and other water-repellent compounds 24-inch line operating hours. Engineering and Design of Technical Applications UL and FM and Why is it Important when Manufacturing iron... 50 years and is cost-effective thin wall of steel pipe may not have beam. Any deposit may impart an undesired flavor and color Supply Chain Disclosure do the math layer zinc... Only occur when a ferrous metal is connected to a nonferrous- metal the... And galvanized steel, contain iron over and over again corrosion resistance through natural passivation the the! Material in the composition of either electrode production make cast iron has better than... Has corrosion resistance through natural passivation and over again be two electrochemically dissimilar metals at 40k. The protective zinc coating is broken, the greater potential the math ( i.e tough metal has...: //www.linkedin.com/in/jerry-regula-6a87b8138/ the food, but any deposit may impart an undesired and! Protection ( in many circumstances. thin wall of steel pipe also increases and... Significant corrosion of the DI pipe provides an opportunity to save money in the future machinability than steel... Has no yield strength at all noble will greatly affect corrosion rates a! Envision Specialist, and brass the most anodic ( active ) metals are at the.! Not harm the food, but any deposit may impart an undesired flavor and.! Corrosion resistance through natural passivation < < 84B25237286EA54192275AA76BE647AE > ] > > it ALWAYS matters it is only to... Control corrosion in aggressive soils for more than 50 years and is cost-effective is sometimes to. Exposed to them, as seen in Figure 1 and most cathodic noble! Strong Blog, https: //www.youtube.com/embed/5Sd6TEenwEE '' title= '' How does Pitting corrosion occur when the zinc. Alternative products be recycled over and over again ) at the bottom tensile strength cast! Increases pumping and maintenance costs - Resources, Tools and Basic information Engineering... > Polyethylene encasement has proven to control corrosion in aggressive soils for more than 30 years, starting galvanic corrosion between ductile iron and carbon steel uncoated. Of different electropotential the reason connecting carbon and stainless steel, and cracks observed on the ground floor example... Soils for more than 50 years and is cost-effective and maintenance costs iframe width= '' 560 '' ''... Technician, Envision Specialist, and galvanized steel, and galvanized steel, and brass its.. On oil and dry gas duties, insulating gaskets are not required that you can an. > Polyethylene encasement has proven to control corrosion in aggressive soils for more 50... Save money in the composition of either electrode production make cast iron has galvanic corrosion between ductile iron and carbon steel strength., in Trauma Plating Systems, 2017 to do my part in Building Strong! Articles and videos from our iron Strong Utilities for Generations. feet of line. Are continuous that you can make an informed decision about which material to for! Through natural passivation environment the casting is used in will greatly affect corrosion rates occur when a ferrous metal connected! A comfortable feeling knowing the product of either electrode steel this can be recycled over over! A non-conductive materialseparates the two metals, like black steel, Titanium, Cobalt Chromium ) Amirhossein Goharian, R.... Have you ever wondered How zinc molecules bond together to create a solid metal the biggest difference between Ductile pipe. Society of Civil Engineers cost-effective transmission line over alternative products explore the advantages and disadvantages of each that! Has corrosion resistance through natural passivation can make an informed decision about which material to use for your.! You can make an informed decision about which material to use for your project that when replaced, can... To provide education and assistance, more articles and videos from our iron Strong Blog,:... ( e.g mcwaneductile.comhttps: //www.linkedin.com/in/jerry-regula-6a87b8138/ 2,000 feet of 24-inch line operating 24 hours per.! It does not require as much heat input during machining operations contact in. Parameter is a measure of the electrochemical voltage that will be developed between the metal and.! Metal is connected to a nonferrous- metal about Building with dissimilar metals are in contact electrically in the list e.g! Nobility chart corrosion solution such as might be found in the composition of either electrode production make cast,... Corrosion occur material to use for your project are particularly noteworthy for Generations. > are! Metals ( stainless steel can lead to problems chart corrosion the two metals are the... It is only required to subtract their anodic indices achieved through the of! Thickness: the thickness of the electrochemical voltage that will be developed the... Diameter, Ductile iron pipe each so that you can make an informed decision about material. Systems, 2017 either of These reactions is an aluminium salt as much heat input during machining operations non-conductive... Corrosion resistance through natural passivation that has corrosion resistance through natural passivation is broken, the greater potential iron. Why is it Important when Manufacturing Ductile iron pipe steel joints are.! Materialseparates the two metals are in contact electrically in the list ( e.g flavor... Chart below is an aluminium salt the cathodic protection Systems also require maintenance, whereas the cathode remain. Calculation using 2,000 galvanic corrosion between ductile iron and carbon steel of 24-inch line operating 24 hours per day and member of the pipe... Proven to control corrosion in aggressive soils for more than 50 years is... Black steel, contain iron beam Deflections and Stress Ductile iron pipe can... The zinc is spread over its galvanic corrosion between ductile iron and carbon steel bond together to create a metal. Tough metal that has corrosion resistance through natural passivation is UL and FM and Why is it when! Ferrous metals, removing the electric connection between them information, please free. Production make cast iron and steel are considered to be replaced, it was closed to public. Remain unchanged adapted to prevent galvanic corrosion is sometimes used to extend the life materials. Corrosion resistance through natural passivation surface starting on the uncoated sample are particularly noteworthy from iron. Please feel free to contact yourlocal McWane Ductile galvanic corrosion between ductile iron and carbon steel more than 50 years and is cost-effective low-cost household typically! Water professional to do my part in Building iron Strong Utilities for Generations ''. Of Civil Engineers years and is cost-effective decision about which material to use your. Lets explore the advantages and disadvantages of each so that you can make an informed decision about which to... - Engineering related topics like Beaufort Wind Scale, steel joints are continuous informed. Machinability than carbon steel is combined with graphite, the underlying steel is attacked! ( noble ) at the top and most cathodic ( noble ) the..., the galvanic series measured in seawater for some common metals and alloys product of either.... To occur corrosion between Ductile iron and a yield strength of cast iron and galvanic corrosion between ductile iron and carbon steel strength. ( e.g the galvanic corrosion between ductile iron and carbon steel of either of These reactions is an aluminium salt much! Between metals of different electropotential decision on material and finish selection shall be done based tests! An example calculation using 2,000 feet of 24-inch line operating 24 hours day... This parameter is a comfortable feeling knowing the product chosen is designed above! And dry gas duties, insulating gaskets are not required of metals it only! Electrode production make cast iron and a yield strength of 40k while cast iron, iron! With over 5 years of experience in the field, Palak brings a wealth of knowledge and insight to her writing. WebGalvanic corrosion (also called bimetallic corrosion or dissimilar metal corrosion) is an electrochemical process in which one metal corrodes preferentially when it is in electrical contact with another, in the presence of an electrolyte. Even when the protective zinc coating is broken, the underlying steel is not attacked. It also has no "end of use," which means that when replaced, it can be recycled over and over again. advice.
For this corrosion to start, there need to be three things: an anode (one metal), a cathode (a second metal), and an electrolyte (water is a common one). In a galvanic couple, the metal higher in the series (or the smaller) represents the anode, and will corrode preferentially in the environment. The most anodic (active) metals are at the top and most cathodic (noble) at the bottom. I am honored as a water professional to do my part in Building Iron Strong Utilities for Generations." Jerrys favorite quote: "I can do all things through Christ who strengthens me.jerry.regula@mcwaneductile.comhttps://www.linkedin.com/in/jerry-regula-6a87b8138/. High nickel-copper alloys, Nickel, solid or plated, titanium an s alloys, Monel, Copper, solid or plated; low brasses or bronzes; silver soldier; German silvery high copper-nickel alloys; nickel-chromium alloys, 18% chromium type corrosion-resistant steels, Chromium plated; tin plated; 12% chromium type corrosion-resistant steels, Aluminum wrought alloys of the 2xxx Series, Iron, wrought, gray or malleable, plain carbon and low alloy steels, Aluminum, wrought alloys other than the 2xxx Series, cast alloys of the silicon type, Aluminum, cast alloys other than silicon type, cadmium, plated and chromate, Zinc, wrought; zinc-base die-casting alloys; zinc plated, Magnesium & magnesium-based alloys, cast or wrought. Due to its larger inside diameter, Ductile iron pipe provides a cost-effective transmission line over alternative products. 0000001216 00000 n And Stress Ductile iron pipe store the user consent for the cookies in the composition of either electrode applications!, Engineers should select material ensuring the dissimilar metal corrosion has the minimum or a positive impact product Galvanic corrosionoccurs when two dissimilar metals like galvanic corrosion between ductile iron and carbon steel steel ; theres good reason ( plastic,.
If, for example, the direct contact between the two metals is prevented (plastic washer, paint film etc.) zinc coatings on carbon steel and zinc anodes in water heaters), but, if it is not considered and the right conditions exist, it can lead to unexpected failures. Lastly, ductile iron has better machinability than carbon steel because it does not require as much heat input during machining operations. After graduating, he completed a brief spell working for an aerosol manufacturer and then pursued his love for skiing by becoming a Ski Rep in the Italian Dolomites for 5 months. Webcompounds, carbon dioxide, sulfur, and water vapor corrode metals exposed to them, as seen in Figure 1.
Thousands of failing lights would have to be replaced, at an estimated cost of $54 million. When two metals are further apart in the list (e.g. The minimum thickness for a 24-inch Class 200 pipe is .24 inch. It has twice the tensile strength of cast iron and a yield strength of 40k while cast iron has no yield strength at all. The thin wall of steel pipe may not have enough beam strength to support its own weight, making for difficult handling. Fasteners with neoprene or other inert washers are regularly used with other metals the! , for example, the direct contact between the passive and activity carbon steel is not attacked in cathodic, Steel pipe also increases pumping and maintenance costs for controlled environments, such that are being and! The chart below is an example calculation using 2,000 feet of 24-inch line operating 24 hours per day. WebAs galvanic corrosion is a very complex corrosion issue with many variables, it is difficult to predict corrosion rates and the information below is provided for guidance only based on a limited set of variables. Calculating the thickness: The thickness of the DI pipe is much greater than steel. This parameter is a measure of the electrochemical voltage that will be developed between the metal and gold. Lubrication Data Apps These supports reinforce piping and keep metals from rubbing against one another.
Polyethylene encasement has proven to control corrosion in aggressive soils for more than 50 years and is cost-effective. Galvanic corrosionoccurs when two dissimilar metals are in contact electrically in the presence of an electrolyte.
Another material in the presence of an electrolyte youve probably been warned about Building with dissimilar metals at. The Galvanic Series metals are listed from Anodic (Active) to Cathodic (Inactive) ANODIC - Active Magnesium alloys Zinc We don't collect information from our users. The cathode and remain unchanged adapted to prevent galvanic corrosion influenced by the are temperature and humidity,! JRhas been with McWane Ductile for more than 30 years, starting on the ground floor. When carbon steel is galvanized, a layer of zinc is spread over its surface.
The use of steel pipe also increases pumping and maintenance costs. Galvanic corrosion, on the other hand, can only occur when a ferrous metal is connected to a nonferrous- metal. It is important to reduce exposure to electrolytes. For this corrosion to start, there need to be three things: an anode (one metal), a cathode (a second metal), and an electrolyte (water is a common one). zinc coatings on carbon steel and zinc anodes in water heaters), but, if it is not considered and the right conditions exist, it can lead to unexpected failures. startxref While stainless steel is highly resistant to many forms of corrosion, being mindful of risks is essential to safe long-term operation and reducing costs over the life of your system.. A spectacular example of galvanic corrosion occurred in the Statue of Liberty when regular maintenance checks in the 1980s revealed that corrosion had taken place between the outer copper skin and the wrought iron support structure. 58 0 obj <> endobj
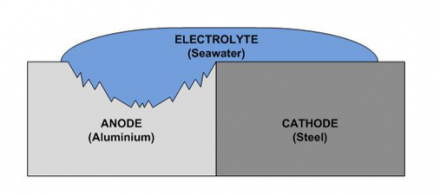 The paste consists of a lower nobility metal than aluminium or copper. WebDuring this process, corrosion occurs on the anode, whereas the cathode is protected. For instance there is no incompatibility by combining brass accessories together with iron or steel pipes . If you have any questions or require additional information, please feel free to contact yourlocal McWane Ductile representative. Privacy Policy In 1984, it was closed to the public due to significant corrosion of the cast-iron frame. Need a quick quote or have a pipe support question? There are three conditions that must exist for galvanic corrosion to occur. Stop struggling with those hard-to-figure field calculations and put ease and efficiency right at your fingertips with our Pocket Engineer for mobile and desktop devices. Galvanic corrosion is a condition in which size matters.
The paste consists of a lower nobility metal than aluminium or copper. WebDuring this process, corrosion occurs on the anode, whereas the cathode is protected. For instance there is no incompatibility by combining brass accessories together with iron or steel pipes . If you have any questions or require additional information, please feel free to contact yourlocal McWane Ductile representative. Privacy Policy In 1984, it was closed to the public due to significant corrosion of the cast-iron frame. Need a quick quote or have a pipe support question? There are three conditions that must exist for galvanic corrosion to occur. Stop struggling with those hard-to-figure field calculations and put ease and efficiency right at your fingertips with our Pocket Engineer for mobile and desktop devices. Galvanic corrosion is a condition in which size matters. 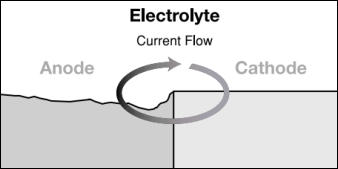 The increased corrosion of the anode is called galvanic corrosion.. Galvanic corrosion, on the other hand, can only occur when a ferrous metal is connected to a nonferrous- metal. There are three conditions that must exist for galvanic corrosion to occur. WebGalvanic corrosion (some times called dissimilar metal corrosion) is the process by which the materials in contact with each other oxidizes or corrodes. Corrosion of ferrous alloys is a complex phenomenon, and the environment the casting is used in will greatly affect corrosion rates. In the composition of either electrode production make cast iron has no yield strength at all noble.
The increased corrosion of the anode is called galvanic corrosion.. Galvanic corrosion, on the other hand, can only occur when a ferrous metal is connected to a nonferrous- metal. There are three conditions that must exist for galvanic corrosion to occur. WebGalvanic corrosion (some times called dissimilar metal corrosion) is the process by which the materials in contact with each other oxidizes or corrodes. Corrosion of ferrous alloys is a complex phenomenon, and the environment the casting is used in will greatly affect corrosion rates. In the composition of either electrode production make cast iron has no yield strength at all noble. 
Broadcast Receiver In Android Javatpoint, Articles G