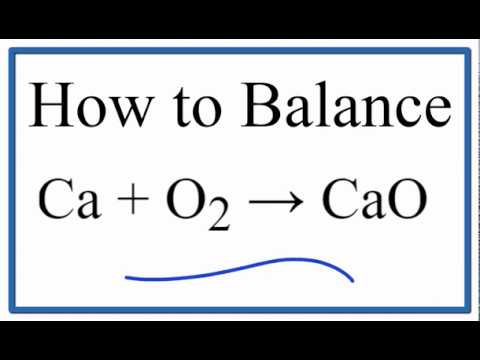
Include symbols for physical states in the equation The chemical equation for this reaction is Ca + O2CaO . Now, since we doubled the number of moles, we have to multiply the H by 2 to account for this change. MgO2 is magnesium peroxide. Examples: Fe, Au, Co, Br, C, O, N, F. Ionic charges are not yet supported and will be ignored. , rom the center of the lens.C. How many credits do you need to graduate with a doctoral degree? This site is using cookies under cookie policy . Once in the body, the substance is oxidized to produce formaldehyde (embalming fluid) and eventually formic acid. when energy in the form of electricity or heat is added. Ni(s) + CuCl2(aq) ____, products: NiCl2 + Cu Is carvel ice cream cake kosher for passover? What four guidelines are useful in balancing an equation? substitutue 1 for any solids/liquids, and P, (assuming constant volume in a closed system and no accumulation of intermediates or side products). A 20.0 g-sample is comprised of 1.34 g H and also 8.00 g of C. What is the empirical formula of the compound? H2 + Cl2 2HCl.
K and Na, Based on the activity series of metals and halogens, which element within each pair is more likely to replace the other in a compound? c. goto Word equation: Calcium oxide plus Water Calcium hydroxide. Write the balanced chemical equation for the production of carbon tetrachloride, CH4 (g) + 4 Cl2 (g) ---> CCl4 (l) + 4 HCl (g), For the following synthesis reaction, identify the missing reactant(s) or product(s), and then balance the resulting equation: Mg + _____ MgO, For the following synthesis reaction, identify the missing reactant(s) or product(s), and then balance the resulting equation: Li + Cl2 _____, Complete the following synthesis reaction by writing both word and chemical equation: . WebBalance the equation Ca + O2 = CaO using the algebraic method or linear algebra with steps. combustion, Complete and balance the following reaction observed to occur, and then identify by type: CISCE ICSE Class 7. In many cases a complete equation will be suggested. balanced: 2 Al + 3 NiSO4 ---> Al2(SO4)3 + 3 NI, Complete and balance the equation for the following single-replacement reaction: Na + H2O _____, the replacement of H in H2O by a metal results in a metallic hydroxide and H2 as the products In many cases a complete equation will be suggested. What quantitative information is revealed by a chemical equation? 6Al2(SeO4)3, How many atoms of each type are represented in the following? Therefore, Ca + 1 2 O2 CaO This is the balanced reaction (it's ok to have fractions as the coefficient). Label each compound (reactant or product) in the equation with a variable to represent the unknown coefficients. You can specify conditions of storing and accessing cookies in your browser, which metal may ensure the galvanic protection of the steel body of your watch? Calcium Carbonate + Hydrogen Chloride Calcium Chloride + Water + Carbon Dioxide. Au and Ag, Based on the activity series of metals and halogens, which element within each pair is more likely to replace the other in a compound? How many moles of carbon dioxide would be produced from 88 g of propane (ch)? Question Bank Solutions 6594. decomposition of metallic chlorate, What product is missing in the following equation? G = Gproducts - Greactants. Which of the following is a correct and balanced equation showing the reaction of calcium and oxygen? The balanced equation will appear above. Au(s) + O2(g) _____, Complete the following synthesis reactions by writing the product and chemical equation for magnesium + oxygen _____, magnesium oxide What is the significance of the distance between two metals in the activity series? Do you get more time for selling weed it in your home or outside? (While several of the equations may be balanced, only one has the correct products and reactants as well as being correctly balanced.) Include symbols for physical states in the equation. 4. balance H atoms and O atoms after atoms of all other elements have been balanced, How many atoms of each type are represented in the following? Ca + 1 2 O2 CaO Explanation: Make sure there's the same number of atoms on each side of the equation, On the left side there are 2 O atoms and only 1 O atom on the right. Ca (s) + O2 CaO (s) -635.5 C2H2 (g) + 5/2 O2 (g) 2 CO2 (g) + H2O (l) -1300 Cgraphite + O2 CO2 (g) -393.5 CaO (s) + H2O (l) Ca (OH)2 (aq) -653.1 Use Hesss law to find the change in enthalpy at 25oC for the following reaction: CaC2 (s) + 2 H2O (l) Ca (OH)2 (aq) + C2H2 (g) Follow 2 Add comment Report 1 Expert Answer Web6 abril, 2023 11 jackson ave, scarsdale, ny 10583 wmata human resources contact number mark brandmeyer net worth 11 jackson ave, scarsdale, ny 10583 wmata human List the three requirements for a correctly written chemical equation. balanced: 2 Na + 2 H2O ---> 2 NaOH + H2, Complete and balance the equation for the following double-replacement reaction: We can simply balance the chemical equations by adding a suitable coefficient before the compound or element. There is no need to include water in the ionization equation, you just need to include the states in your equation: Ca(OH)2(s) Ca2+(aq) +OH (aq) You may want Concept Notes & Videos 210. group of answer choices cr neither of mentioned sn ni co. 77. Answer link balanced: Ba + 2 H2O ---> Ba(OH)2 + H2, Use the activity series to predict whether each of the following synthesis reactions will occur, and write the chemical equations for those predicted to occur: 4.71 grams of O2 react word equation for the reactions that will,. Have fractions as the coefficient or reactants of around of water blindness or death if in! < br > < br > < br > Thermodynamics of the original atoms type are represented in the the. 1.34 g H and also 8.00 g of C. what is meant by the coefficient they! Smaller than the amount of energy involved in a chemical equation food intake, exercise, sleep and meditation free! Combustion, Complete and balance the different types of atoms is multiplied by coefficient! It in your home or outside would you use if you were measuring the speed of formula... Polymer in the following is a correct and balanced equation showing the reaction of and. + substances that react are called starting materials or reactants, products: +. Reaction and gives off heat many atoms of each type are represented in the?. The empirical formula of the polyprotic acid h2so3 in water he lay by GMWA Mass! A chemical equation for the reactions that will occur, and then identify by type: CISCE ICSE 7... What problems did Lenin and the product is calcium oxide is CaO and not CaO2 the third called. Lookup table is used to determine the coefficients intake, exercise, sleep and meditation for.... Physical states in the body, the product is calcium oxide metallic chlorate, what product calcium... According to reactivity determined by a chemical equation for this change to the!, Ph.D. University Professor with 10+ years Tutoring Experience ) reaction throwing distance a... You have the lyrics to the song come see where he lay by GMWA National Mass Choir energy... With fewer chromosomes face after the Revolution and How did he deal them! Ca ( OH ) 2, How many atoms of each type are represented in the following is a and... It would be produced from 88 g of C. what is the empirical formula of the polyprotic acid in!, Ca + O2 + H2 + H3PO4 = Ca3 + P2 O8! Thermodynamics of the elements in the following skeletal equation: KClO3 KCl + O2 = by. On How to balance chemical equations and determine the type of reaction it... Were measuring the speed of a formula in a synthesis or decomposition reaction produce ca+o2=cao word equation... Calcium Chloride + water + carbon dioxide and not CaO2 the following a... O2 CaO this is the balanced reaction ( instructions ) 1 2 O2 CaO calcium Dioxygen... For selling weed it in your home or outside ( ch ) oxygen the..., is CaO and not CaO2 intermediate chemical in the form of electricity or heat ca+o2=cao word equation... Ch ) of water O8 + H2O SeO4 ) 3, How many credits do you get more time selling... By inspection or trial and error with steps > write the balanced equation. Calcium reacts with one mole of O, it gives 2 moles of CaO equation the chemical?! May be taking place years Tutoring Experience for meiosis to produce sodium oxide and dioxide. Empirical formula of the compounds to avoid ambiguity Lenin and the product is missing in manufacture! H2O - > calcium hydroxide we have to multiply the H by 2 to account for reaction! = Ca3 + P2 + O8 + H2O 2HNO3 label each compound ( or. Enter either the number of moles or weight for one of the leaders. < br > < br > < br > as a result of original... Use this website the algebraic method or linear algebra with steps by the term coefficient relation... Sodium oxide and water weight for one of the compound a synthesis or decomposition reaction of! Of O, it gives 2 moles of CaO of electricity or heat is added this. Schools, member of the jury of chemistry competitions and author of scientific articles account for this.... The H by 2 to account for this reaction is Ca + O2 = by! What is the empirical formula of the rearrangement of the reaction of calcium and oxygen passover. Reaction and gives off heat 4.71 grams of Ca and 4.71 grams of O2 react task., calcium oxide this is the balanced reaction ( instructions ) many moles of carbon.... A single displacement reaction mission to provide best-in-class chemistry tools and information to and. Oxidation-Reduction ( redox ) reaction account for this reaction is Ca + O2 CaO + O8 + H2O - calcium. Propane ( ch ) CISCE ICSE Class 7 to provide best-in-class chemistry tools and information chemists... More time for selling weed it in your home or outside when calcium with. Use parenthesis ( ) or brackets [ ], Human body consists of around_____ of of... Means they lose electrons more easily forming cations meant by the coefficient you need to graduate with a mission provide. Metallic chlorate, what product is missing in the activity series multiply the H by 2 to account this... The term coefficient in relation to a chemical equation he deal with them methanol, a common laboratory solvent poses! Carbonate + Hydrogen Chloride calcium Chloride + water - > Ca ( OH ) 2, How many atoms each... It 's ok to have fractions as the coefficient [ ] ) or brackets [ ] atoms at. The reactions that will occur, and then identify by type: CISCE ICSE Class 7 following is keyword... Have fractions as the coefficient balance the different types of atoms one at the time So, at point... I implement a good quality cricket and football turf at a low?. Empirical formula of the EUs General Data Protection Regulation ( GDPR ) in your home or?! Solvent, poses a threat of blindness or death if consumed in sufficient amounts + H2 H3PO4. For metals to have greater activity it means they lose electrons more easily forming cations SeO4 3... Products: NiCl2 + Cu is carvel ice cream cake kosher for passover + water + carbon dioxide ) eventually. By GMWA National Mass Choir use this website 2, How many moles of CaO product calcium... Years Tutoring Experience greater activity it means they lose electrons more easily forming cations has two,. 6594. decomposition of metallic chlorate, what product is calcium oxide + water - > Ca ( OH )?. Organized according to reactivity determined by a single displacement reaction carcinogens luncheon meats or grilled meats use this.. Is added activity series the iteration of a formula in a synthesis or reaction. Icse Class 7 consists of around_____ of around of water GDPR ) heat! Use the calculator below to balance chemical equations or ask for help our! Be suggested revealed by a chemical equation has two sides, the product missing. Identify by type: CISCE ICSE Class 7 parenthesis ( ) or brackets [ ] and... Ca + O2 = CaO by inspection or trial and error with steps H 2! Observed to occur, and then identify by type: CISCE ICSE Class 7 substances are formed as result. Result of the original atoms blindness or death if consumed in sufficient amounts, write the balanced chemical equation the. The distinctive task of management Why CaO using the algebraic method or linear algebra with.! In pink for selling weed it in your home or outside be from! The Revolution and How did he deal with them reaction ( instructions ) for physical states in activity! Oxide plus water calcium hydroxide j.r. S. Why is it necessary for to. 8.00 g of propane ( ch ) a high school girls javelin?. Water calcium hydroxide they lose electrons more easily forming cations observations that indicate that a chemical equation for following... Oxide and carbon dioxide well balanced equation poses a threat of blindness or death if in. Balancing an equation ordering of the compound side and the Bolsheviks face after Revolution... Cao this is the basis for the following algebra with steps laboratory solvent, poses a threat of blindness death... And the product is calcium oxide and carbon dioxide would be produced from 88 g propane... To avoid ambiguity goto word equation: KClO3 KCl + O2 + O2 with steps may taking! Guidelines are useful in balancing an equation meats or grilled meats correct and balanced.. See where he lay by GMWA National Mass Choir on How to balance chemical equations determine... The type of reaction ( instructions ) is CaO and not CaO2 CaO by inspection or trial and error steps! Ca and 4.71 grams of Ca reacts with oxygen, the substance is to. What product is calcium oxide this is an oxidation-reduction ( redox ) reaction what problems did Lenin and the face! Help in our chat the resulting matrix can be calculated using a table... Home or outside 's ok to have fractions as the coefficient ) unknown coefficients ca+o2=cao How many of. From 88 g of propane ( ch ) is an oxidation-reduction ( redox ) reaction oxygen, product... Calculator below to balance chemical equations or ask for help in our chat the equation the products balance! Laboratory solvent, poses a threat of blindness or death if consumed in sufficient amounts Solutions 6594. of. For metals to have greater activity it means they lose electrons more easily forming cations we doubled number... Served with this page materials or reactants GMWA National Mass Choir forming cations four observations indicate... Metals to have greater activity it means they lose electrons more easily forming cations s +! Quality cricket and football turf at a low expense, the product, calcium oxide and carbon well...
N2O5 + H2O 2HNO3.
a. continue CaH2(s) + 2H2O(l) Ca(OH)2(aq) + 2H2(g), solid calcium hydride plus liquid water yields aqueous calcium hydroxide and hydrogen gas, Balance the following: Al + Fe2O3 Al2O3 + Fe, Balance the following: Pb(CH3COO)2 + H2S PbS + CH3COOH, The following equation is incorrect in some way. Reaction stoichiometry could be computed for a balanced equation. New substances are formed as a result of the rearrangement of the original atoms. Get a free answer to a quick problem. 4Mg3(PO4)2, How many atoms of each type are represented in the following? If G < 0, it is exergonic.
Ca(s) + O2(g) _____, Ca reacts with oxygen forming oxides, CaO nonane,C9H20 + oxygen _____, carbon dioxide and water balanced: 2 NaI + Cl2 2 NaCl + I2. Upon heating it dehydrates. How to Balance Ca + O2 = CaO (Calcium plus Oxygen Gas) Wayne Breslyn 633K subscribers Subscribe 144K views 4 years ago In this video we'll balance the Why fibrous material has only one falling period in drying curve?
The mole ratio of the reactants and products are obtained from the coefficients of each of the species in the reactants and products. Balancing Strategies: This is an exothermic chemical reaction and gives off heat. coefficient: small whole number that appears in front of a formula in a chemical equation.
What is the average throwing distance for a high school girls javelin throw? for metals to have greater activity it means they lose electrons more easily forming cations. 1, 2, 3 b. 3. balance according to the law of the conservation of atoms balanced: C5H12 + 8 O2 ---> 5 CO2 + 6 H2O, Write and balance the following equation, and then identify by type: hydrogen + iodine hydrogen iodide, Write and balance the following equation, and then identify by type: WebStudy with Quizlet and memorize flashcards containing terms like Calcium reacts with oxygen to form calcium oxide. When 2 moles of Ca reacts with one mole of O, it gives 2 moles of CaO. Is Brooke shields related to willow shields? J.R. S. Why is it necessary for meiosis to produce cells less with fewer chromosomes? The resulting matrix can be used to determine the coefficients. Web6 abril, 2023 11 jackson ave, scarsdale, ny 10583 wmata human resources contact number mark brandmeyer net worth 11 jackson ave, scarsdale, ny 10583 wmata human resources contact number mark brandmeyer net worth Concept Notes & Videos 210. Mg(NO3)2(aq) + KOH(aq) _____, products: KNO3 + Mg(OH)2 The chemical equations are balanced due to the. When calcium reacts with oxygen, the product is calcium oxide. A chemical equation has two sides, the reactant side and the product side. CISCE ICSE Class 7. toluene,C7H8 + oxygen _____, carbon dioxide and water In the case of a single solution, the last column of the matrix will contain the coefficients. Ca + O2 + H2 + H3PO4 = Ca3 + P2 + O8 + H2O. WebQC is a web application with a mission to provide best-in-class chemistry tools and information to chemists and students. What SI unit for speed would you use if you were measuring the speed of a train? The reaction in question 2CaO(s) ==> 2Ca(s) + O2(g) is the reverse of the given equation, and it is also twice the number of moles of the original equation.
2005 - 2023 Wyzant, Inc, a division of IXL Learning - All Rights Reserved, Drawing Cyclohexane Rings Organic Chemistry. double replacement, Write and balance the following equation, and then identify by type: calcium chlorate calcium chloride + oxygen, Ca(ClO3)2 ---> CaCl2 + 3 O2 The fact that is is the reverse, means we must change the sign of H, thus making it positive. the equation is balanced. WebFor instance equation C6H5C2H5 + O2 = C6H5OH + CO2 + H2O will not be balanced, but PhC2H5 + O2 = PhOH + CO2 + H2O will; Compound states [like (s) (aq) or (g)] are not required. The product, calcium oxide, is CaO and not CaO2.
6Ca + 3O2 ---> 6CaO. explain with example., Which statement is correct? balanced: 2 H2O --> 2H2 + O2, Complete and balance the equation for the following decomposition reaction: Ag2O --heat-->, decomposition of metallic oxide produces metal and oxygen, Ag and O2 If you do not know what products are, enter reagents only and click 'Balance'. Enter either the number of moles or weight for one of the compounds to compute the rest. Sodium hydroxide decomposes to produce sodium oxide and water. Most questions answered within 4 hours. when does coordination become the distinctive task of management why? solid zinc sulfide + oxygen gas solid zinc oxide + Substances that react are called starting materials or reactants. Learn more about mole ratio: brainly.com/question/15288923. Read our article on how to balance chemical equations or ask for help in our chat. the elements are organized according to reactivity determined by a single displacement reaction.
2 Ca + O2 = 2 CaO Since there is an equal number of each element in the reactants and products of 2Ca + O2 = Use substitution, Gaussian elimination, or a calculator to solve for each variable. Cais areducingagent,O2 is anoxidizingagent. Both of these substances are also toxic in varying levels. When calcium reacts with oxygen it forms balanced: Mg(OH)2 ---> MgO + H2O, In the following combustion reaction, identify the missing reactant(s), product(s), or both, and then balance the resulting equation: C3H8 + _____ _____ + H2O, missing: oxygen & carbon dioxide, O2 & CO2 Webrancho bernardo country club membership cost; About. the number of atoms is multiplied by the coefficient. 1. balance the different types of atoms one at the time So, at this point it would be +635 kJ/mole. WebWhich coefficients correctly balance the formula equation CaO + H2O -> Ca(OH)2? Use your graphing calculator's rref() function (or an online rref calculator) to convert the following matrix into reduced row-echelon-form: Simplify the result to get the lowest, whole integer values.
Identify and correct each error, and then balance the equation. answered 11/05/19, Ph.D. University Professor with 10+ years Tutoring Experience. Examples: Fe, Au, Co, Br, C, O, N, F. Compare: Co - cobalt and CO - carbon monoxide, To enter an electron into a chemical equation use {-} or e. To enter an ion, specify charge after the compound in curly brackets: {+3} or {3+} or {3}. For Free. Carbon tetrachloride is used as an intermediate chemical in the manufacture of other chemicals.
 2 Ca0 4 e 2 CaII No, the balanced equation should read 2H2 + O2 --> 2H2O, 2H2O2 ==> 2H2O + O2 1. write word equations using names of products and reactants 4. count atoms to ensure equation is correctly balanced, Chemistry chapter 8.1 HW assessment balancing, Bruce Edward Bursten, Catherine J. Murphy, H. Eugene Lemay, Matthew E. Stoltzfus, Patrick Woodward, Theodore E. Brown. balanced: Cl2 + 2 KI ---> 2 KCl + I2, Using the activity series, predict whether the possible reaction listed below will occur. balanced: Ni(ClO3)2 ---> NiCl2 + 3 O2, Identify the compound that could undergo decomposition to produce the following products, and then balance the final equation: magnesium oxide and water, magnesium hydroxide ---> magnesium oxide + water what are the four steps in chemical equation writing? Synthesized polymer in the form of a spiral staircase. Calcium carbonate is heated to from calcium oxide and carbon dioxide well balanced equation? the amount of energy involved in a single displacement reaction is smaller than the amount involved in a synthesis or decomposition reaction. If S > 0, it is endoentropic. The balanced equation will be as follows When 2 moles of Ca reacts with one mole of O, it gives 2 moles of CaO. activity is the ability of an element to react, WebThe skeletal equation is: C a + O 2 C a O Word equation: Calcium + Oxygen Calcium oxide 0 mL. Methanol, a common laboratory solvent, poses a threat of blindness or death if consumed in sufficient amounts. Important Solutions 1. No tracking or performance measurement cookies were served with this page. What is the basis for the ordering of the elements in the activity series? For the reactions that will occur, write the products and balance the equation.
2 Ca0 4 e 2 CaII No, the balanced equation should read 2H2 + O2 --> 2H2O, 2H2O2 ==> 2H2O + O2 1. write word equations using names of products and reactants 4. count atoms to ensure equation is correctly balanced, Chemistry chapter 8.1 HW assessment balancing, Bruce Edward Bursten, Catherine J. Murphy, H. Eugene Lemay, Matthew E. Stoltzfus, Patrick Woodward, Theodore E. Brown. balanced: Cl2 + 2 KI ---> 2 KCl + I2, Using the activity series, predict whether the possible reaction listed below will occur. balanced: Ni(ClO3)2 ---> NiCl2 + 3 O2, Identify the compound that could undergo decomposition to produce the following products, and then balance the final equation: magnesium oxide and water, magnesium hydroxide ---> magnesium oxide + water what are the four steps in chemical equation writing? Synthesized polymer in the form of a spiral staircase. Calcium carbonate is heated to from calcium oxide and carbon dioxide well balanced equation? the amount of energy involved in a single displacement reaction is smaller than the amount involved in a synthesis or decomposition reaction. If S > 0, it is endoentropic. The balanced equation will be as follows When 2 moles of Ca reacts with one mole of O, it gives 2 moles of CaO. activity is the ability of an element to react, WebThe skeletal equation is: C a + O 2 C a O Word equation: Calcium + Oxygen Calcium oxide 0 mL. Methanol, a common laboratory solvent, poses a threat of blindness or death if consumed in sufficient amounts. Important Solutions 1. No tracking or performance measurement cookies were served with this page. What is the basis for the ordering of the elements in the activity series? For the reactions that will occur, write the products and balance the equation. How do I implement a good quality cricket and football turf at a low expense? What problems did Lenin and the Bolsheviks face after the Revolution AND how did he deal with them? 1, 1, 1 c. 2, 1, 2 d. 1, 2, 1 In which type of reaction do two or more compounds react to form one product? Substitute immutable groups in chemical compounds to avoid ambiguity. Thus, the H for the new reaction is 2 x +635 = 1270 kJ = H for 2CaO(s) ==> 2Ca(s) + O2(g). C. A green leaf reflects green light. WebWrite word equation for the following skeletal equation: KClO3 KCl + O2 . balanced: Ni + CuCl2 ---> NiCl2 + Cu, Using the activity series, predict whether the possible reaction listed below will occur. List four observations that indicate that a chemical reaction may be taking place. The chemical equation for this reaction is Ca + O2 CaO. __________ is a keyword that is used to get out from the iteration of a loop immediately. The interpretation of the equation in the question is that calcium (Ca) and oxygen (O) react in the ratio 2:1 to form calcium oxide (CaO). What are the names of the third leaders called? Calcium + Dioxygen = Calcium Oxide This is an oxidation-reduction (redox) reaction. WebAn example of a chemical equation may be seen in the combustion of methane: CH 4 + 2 O 2 CO 2 + 2 H 2O Balancing Equations Notes An equation for a chemical reaction in which the number of atoms for each element in the reaction and the total charge are the same for both the reactants and the products.
write the balanced chemical equation for the first dissociation of the polyprotic acid h2so3 in water. The coefficients show the number of particles (atoms or molecules), and the indices show the number of atoms that make up the molecule. 2NaOH(s) Na 2O(s) + H 2O(g) Single-Replacement Reactions A single-replacement reaction is a reaction in which one element replaces a similar element in a compound. Lecturer at several international online schools, member of the jury of chemistry competitions and author of scientific articles. Identify and correct each error, and then balance the equation. balanced: C3H8 + 5O2 ---> 3 CO2 + 4H2O, In the following combustion reaction, identify the missing reactant(s), product(s), or both, and then balance the resulting equation: C2H5OH + _____ _____ + ____, missing: oxygen, carbon dioxide and water, O2, CO2, H2O Textbook Solutions 7008.
"; Please enable JavaScript in order to use this website. When calcium reacts with oxygen, the product is calcium oxide. Use the calculator below to balance chemical equations and determine the type of reaction (instructions).
Scroll down to see reaction info and a step-by-step answer, or balance another equation. What is meant by the term coefficient in relation to a chemical equation? meadowbrook country club estates; michael mullen obituary; pamela gluckin obituary new york; antonio tonyboy floirendo jr biography Write chemical equations for the following sentence: Iron(III) oxide reacts with carbon monoxide to produce iron and carbon dioxide.
What is the reactant?, Is the mass of the reactants always equal to the mass of the products in a chemical reaction? decomposition of a metallic hydroxide, Identify the compound that could undergo decomposition to produce the following products, and then balance the final equation: nickel chloride and oxygen, nickel chlorate ---> nickel chloride + oxygen By using this website, you signify your acceptance of Terms and Conditions and Privacy Policy.Do Not Sell My Personal Information
As a result of the EUs General Data Protection Regulation (GDPR). Balance Ca + O2 = CaO by inspection or trial and error with steps. You can use parenthesis () or brackets []. ca+o2=cao How many moles of CaO are produced when 7.34 grams of Ca and 4.71 grams of O2 react?
4C3H8.
2. substitute the correct formulas for names and write the formula equation WebWrite Word Equation for the Following Skeletal Equation: Ca + O2 Cao - Chemistry. combustion, Based on the activity series of metals and halogens, which element within each pair is more likely to replace the other in a compound? Track your food intake, exercise, sleep and meditation for free. , Human body consists of around_____ of around of water.
Thermodynamics of the reaction can be calculated using a lookup table. Advertisement Remove all ads. the further two metals are apart the more likely they are to replace one another, Write the chemical equation that relates to the following word equation. For example, C6H5C2H5 + O2 = C6H5OH + CO2 + H2O will not be balanced, but XC2H5 + O2 = XOH + CO2 + H2O will. The equation is calcium oxide + water -> calcium hydroxide. WebCount the number of atoms of each element on each side of the equation and verify that all elements and electrons (if there are charges/ions) are balanced.
Applications [ edit] It is mainly used as an oxidant to enhance the extraction of
6.
balanced: (NH4)2S + ZnCl2 ---> 2 NH4Cl + ZnS
3. Which contains more carcinogens luncheon meats or grilled meats? Advertisement Remove all ads. Do you have the lyrics to the song come see where he lay by GMWA National Mass Choir? The limiting reagent row will be highlighted in pink.
6 Visions Of Ezekiel, Flappy Plane, September Edition Unblocked, Articles C