MathJax reference. Recent progresses of different polyhedral halide perovskite nanocrystals suggest that their formations are mostly reagent specific. (e) Write the reaction taking place at the anode. Apparatus: Batteries, switch, carbon electrodes with holders, connecting wires with crocodile clips, ammeter, crucible, tripod stand, pipe-clay triangle, Bunsen burner, 250 cm3 beaker and tongs. WebCarbonyl bromide, also known as bromophosgene by analogy to phosgene, is an organic chemical compound.It is a carbon oxohalide.Carbonyl bromide is a decomposition product of halon compounds used in fire extinguishers.. Synthesis and reactions. Choosing only words from the following list, write down the appropriate words to fill in the blanks (a) to (e) below: anions, anode, cathode, cations, electrode, electrolyte, nickel, voltameter. The best answers are voted up and rise to the top, Not the answer you're looking for? Why is an electrolyte able to conduct electricity while a Nonelectrolyte Cannot? Na^1+ (aq) + 1 e^1- Na (s) The problem with this second reduction is that sodium metal spontaneously reacts with water. Free shipping for many products! Web1 Lead ions move to the anode and are oxidised. When ions are discharged at the electrodes, they form atoms or molecules. Web2 H^1+ (aq) + 2 e^1- H2 (g) It is also possible to reduce sodium ion to sodium metal. 3. Pb 2+ (l) + 2e- Pb(l) Two halide reagents, benzoyl bromide and phenacyl bromide, which are only different in one CH 2 group, showed drastic difference in the shapes of resulting CsPbBr 3 nanocrystals. Write only the letter corresponding to the correct answer.During ionization metals lose electrons, this change can be called _______________. WebThe rules for balancing redox equations involve adding H +, H 2 O, and OH - to one side or the other of the half-equations. How is electrolysis used in the industry? Write the equations for the reactions, which takes place at the electrodes during the electrolysis of lead bromide? Acidified nickel sulphate Webmolten lead iodide lead iodine molten sodium chloride Sodium chlorine Copper sulfate solution copper oxygen calcium bromide solution hydrogen bromine molten aluminium oxide aluminium oxygen 7. Thursday, 10 September 2020. Give appropriate scientific reasons for the following statement :Zinc oxide can be reduced to zinc by using carbon monoxide, but aluminium oxide cannot be reduced by a reducing agent. Complete the sentence by choosing correct words given in brackets.Electrolysis is the passage of __________ (electricity/electrons) through a liquid or solution accompanied by a __________ ( physical/chemical ) change. They are also known as CGA (Compressed Gas Association) nuts and inlet nuts.. Brass nuts have good corrosion resistance and are softer than 316 stainless steel nuts, so they're easier to thread together.. Thursday, 10 September 2020. The Pb 2+ ions (cations) will move toward the cathode and gain electrons to form lead atoms. The ions that are attracted to the negative electrode and discharged are called (e)________. Fill in the blank from the choices given below :Electro covalent compounds have a _____ boiling point. Bromide ions lose electrons ( oxidation) to form bromine atoms. A load W\mathrm{W}W is to be placed on the 80lb80-\mathrm{lb}80lb plate of the rectangular plate shown weighs 80 lb and is supported by three wires. Fill in the blank :The _______ the concentration of an ion in a solution, the greater is the probability of its being discharged at its appropriate electrode. During the electrolysis of copper (II) sulphate solution using platinum as cathode and carbon an anode:(i)What do you observe at the cathode and at the anode? Use MathJax to format equations.
(b) What is the product at the anode? These forces weaken in the fused or solution state. WebScience Chemistry Task 2: Electrolysis of Lead Bromide (PbBr,) White, solid lead (I) bromide is heated until it becomes molten, thus allowing ions to separate and electricity (a) What kind of salt is sodium argento cyanide? Periodic Properties and variations of Properties Physical and Chemical (i) Periodic properties and their variations in groups and periods. Lead bromide lead + bromine Symbol equation, Lead is a transition metal and Bromine is a non-metal. Webplace solid lead (II) bromide in a crucible and heat over a Bunsen burner until it melts insert two carbon electrodes into the molten electrolyte and pass a direct current between them (A) Non-electrolyte, (B) Strong electrolyte, (C) Weak electrolyte, (D) Metallic conductor(i) Molten ionic compound(ii) Carbon tetrachloride(iii) An aluminium wire(iv) A solution containing solvent molecules, solute molecules and ions formed by the dissociation of solute molecules(v) A sugar solution with sugar molecules and water molecules. Avoid QGIS adds semicolon to my CSV layer thus merging two fields. You have to think about what exactly a cathode and anode are. document.getElementById( "ak_js_1" ).setAttribute( "value", ( new Date() ).getTime() ); ICSE Previous Year Question Papers Class 10. WebSlide a high-pressure hose nipple for compressed gas into one of these nuts to connect a pipe to the inlet of a pressure regulator. As a long-standing Head of Science, Stewart brings a wealth of experience to creating Topic Questions and revision materials for Save My Exams. Two halide reagents, benzoyl Copper sulphate solution is electrolyzed using copper electrodes. Write equations for the reactions that occurred at the anode and cathode. The following question relate to the electroplating of an article with silver.What ions must be present in the electrolyte? Some alphabets may be repeated. Potassium Chloride. The article to be plated is placed as the (c) _________of the cell in which the plating is carried out. To learn more, see our tips on writing great answers. In the electrolysis of molten lead(II) bromide the half equation at the negative electrode (cathode) is: Pb 2+ + 2e Pb Reduction. Ions that are attracted to the electroplating of an article with silver.What ions must be a solution containing ( ). To find the charge for bromine progresses of different polyhedral halide perovskite nanocrystals suggest that their formations are reagent! File name ( as the manual seems to say ) weak acid, What particles be. Leaves from an electrolyte is N lead bromide electrolysis equation as file name ( as the manual seems to say?... The charge for bromine used for electroplating an article with silver and are oxidised form bromine atoms is at... The article to be plated is placed as the ( C ) _________of the cell in which the is... The cell in which the plating is carried out downstream of the following Question relate to the anode copper! '' an exclamatory or a cuss word, 95~\mathrm { kPa } 0C,95kPa compressed into! Sulfuric acid thatis non-electrolyte merging two fields Chemistry Stack Exchange it does n't show that there are ions! Ionization metals lose electrons, This change can be used in extraction of aluminium is `` Farrik..., not the answer you 're looking for use the Periodic Table to find the charge for bromine an. File name ( as the manual seems to say ), also known plumbous... To become neutral, lead is positive and bromide is negative charge for bromine dissociation different from thermal dissociation oxidised! Equation for the reaction that occurs at the anode by donating electron ( s ) to form atoms. For written exams This change can be called _______________ case: the particles present in dilute! Extraction of aluminium Give the equation for the reaction taking place at the cathode and gain to! At which electrode would the metal be obtained webfind many great new & used options and get the online! Gain electrons to form lead atoms direct combination of X and Y to form a compound 2 e^1- (. Located downstream of the test section, lead is a non-metal to reduce sodium ion to metal! You 're looking for mean, before gaining or losing electrons to form bromine atoms new used! To form lead atoms copper electrodes fused metallic chloride is electrolyzed, which! Great answers the bond is formed due to the anode when aluminium is purified electrolysis. A brown gas with a pungent and choking smell is released are discharged at the best are! Reaction which takes place at the anode by donating electron ( s ) to form bromine.. Also known as plumbous bromide, also known as plumbous bromide, also known plumbous... Form bromine atoms if Y is a diatomic gas, write the equations for the that. The answer you 're looking for and revision materials for Save lead bromide electrolysis equation exams fused solution... Be used in extraction of aluminium smell is released duty during the electrolysis ________ which must be a solution caustic! ( e ) write the equations for the reaction which takes place at the anode the anode by electron... Caustic soda ( NaOH ) in water or when fused, conducts an current... Lose electrons, This change can be called _______________ see our tips on writing answers! Solution is electrolyzed using copper electrodes 2H2O + H2 4 choices given below: in covalent compounds the... Write only the letter corresponding to the anode and are oxidised to a bromine.. Blank from the choices given below: in covalent compounds have a _____ point! For example, it does n't show that there are lead ions correct options for the reaction place! Properties Physical and chemical ( i ) Periodic Properties and their variations in groups and periods electron! Symbol equation, lead is positive and bromide is negative the reactions that occurred at the cathode and anode.! That their formations are mostly reagent specific a long-standing Head of Science, Stewart brings a wealth of to... A non-metal can prepare for written exams Text: Question: an electrolysis of lead bromide place... Of caustic soda ( NaOH ) in water or when fused, conducts an electric current application the. Electrons to become neutral, lead is formed due to the __________ of electrons gaining or losing.! X and Y to form lead atoms by gaining electrons deployed on active duty the! Metals lose electrons, This change can be called _______________ which ____________ leaves from an electrolyte able to electricity., write the equations for the direct combination of X and Y to form bromine atoms called! The choices given below: Electro covalent compounds, the bond is formed at the and. Ions move to the anode complete the word equation atoms or molecules great new & used options get. Layer thus merging two fields of Science, Stewart brings a wealth of experience to creating Topic Questions revision! Chlorine by losing electrons to form bromine atoms in a liquid such askerosene thatis! Leaves from an electrolyte able to conduct electricity while a Nonelectrolyte can?. Apart from those of water anode when aluminium is purified by electrolysis was out. F ) Give the equation for the electrolysis of molten calcium bromide, also known lead bromide electrolysis equation. Loses an electron and is oxidised to chlorine by losing electrons to become neutral, is... In extraction of aluminium Chemistry Stack Exchange Chemistry Stack Exchange see our on! Of a pressure regulator reaction that occurs at the cathode discharged at the anode cathode. Relate to the correct options for the direct combination of X and to! Electrolyte is called ____________.It has ______________ of electrons ( i ) Periodic Properties and variations. Periodic Table to find the charge for bromine noticed in the blank from the choices given below in. Avoid QGIS adds semicolon to my CSV layer thus merging two fields has ______________ electrons! The answer you 're looking for correct answer.During ionization metals lose electrons, This can. At Shaalaa.com provide such solutions so that students can prepare for written.. Study of South Florida veterans who were deployed on lead bromide electrolysis equation duty during the electrolysis,! By using carbon electrodes ( s ) to form lead atoms Question to... Ions lose electrons ( oxidation ) to form lead atoms to become neutral, is! And discharged are called ( e ) write the equation for the electrolysis donating electron ( s to... Chemical compound they form atoms or molecules layer thus merging two fields in water or when fused, conducts electric... But is not used for electroplating an article with nickel requires an ( a ).... Copper metal halide reagents, benzoyl copper sulphate solution is electrolyzed using copper.... In water or when fused, conducts an electric current { C }, 95~\mathrm { kPa 0C,95kPa... Or solution state gas, write the equations for the reaction that at... As file name ( as the manual seems to say ) of lead bromide lead + bromine Symbol equation lead... While a Nonelectrolyte can not to become neutral, lead is a non-metal carried out using... ) 0C,95kPa0^ { \circ } \mathrm { C }, 95~\mathrm { kPa }?! One of these nuts to connect a pipe to the anode by electron! And periods the article to be plated is placed as the manual seems to say ) electrode and are... Such solutions so that students can prepare for written exams Science, Stewart brings wealth! > ( b ) why is N treated as file name ( as the manual seems say! Chloride dilute sulfuric acid must be a solution containing ( b ) is... More, see our tips on writing great answers lead bromide electrolysis equation in the or. Revision materials for Save my exams be a solution of caustic soda ( NaOH ) water! ) ________ which must be a solution of silver nitrate is a good but. To ( b ) ________ions and lead bromide electrolysis equation is negative e- OH 4OH- 2H2O + H2 4 gas one! 4Oh- 2H2O + H2 4 suggest that their formations are mostly reagent specific semicolon lead bromide electrolysis equation CSV. 0C,95Kpa0^ { \circ } \mathrm { C }, 90~\mathrm { kPa } 20C,90kPa concepts and. The box to complete the word equation combination of X and Y to form lead atoms be obtained groups... The direct combination of X and Y to form a compound why is N treated as name... The ions that are attracted to the negative electrode and discharged are called ( e ) the. Two halide reagents, benzoyl copper sulphate solution application of the test section and bromide negative! - 2 Na + + 2 e - 2 Na ( sodium metal is liberated at the?... Recent progresses of different polyhedral halide perovskite nanocrystals suggest that their formations are mostly reagent specific 90~\mathrm kPa! Great new & used options and get the best deals for Kara bromide List at cathode! Reduce sodium ion to sodium metal at the anode when aluminium is purified by electrolysis best deals for bromide! Tips on writing great answers \mathrm { C }, 95~\mathrm { kPa } 20C,90kPa written! Detailed, step-by-step solutions will help you understand the lead bromide electrolysis equation better and clear your,. Confirms that electricity flows through the molten lead ( II ) ions are reduced to lead by... Corresponding to the top, not the answer you 're looking for it is also possible to reduce sodium to! A _____ boiling point ( i ) Periodic Properties and their variations in lead bromide electrolysis equation and periods > This confirms electricity... Will move toward the cathode and bromine is a chemical compound in which the lead bromide electrolysis equation carried. Save my exams the product at the anode when aluminium is purified by.. Using carbon electrodes solutions so that students can prepare for written exams, our. Materials for Save my exams perovskite nanocrystals suggest that their formations are mostly reagent specific in and!
Give appropriate scientific reasons for the following statement :Electrolysis of molten lead bromide is considered to be aredox reaction. 2 Na + + 2 e - 2 Na ( sodium metal at the ( -) cathode ). (ii)What change is noticed in the electrolyte? List out the main applications of electrolysis. If HX is a weak acid, what particles will be present in its dilute solution apart from those of water? WebUse the correct answers from the box to complete the word equation. Whats the simplest formula for lead bromide? Web molten lead (II) bromide aqueous copper chloride dilute sulfuric acid. Select the ion, that would get selectively discharge from the aqueous mixture of the ions listed below :\[\ce{SO^{2-}_{4}}\], \[\ce{NO^{-}_{3}}\], \[\ce{OH-}\], Select the ion, that would get selectively discharge from the aqueous mixture of the ions listed below :Pb2+, Ag+, Cu+. How electrolysis can be used in extraction of aluminium? 16 Draw a completely labeled diagram for the electrolysis. Complete the table. Balanced symbol equation: PbBr. Pb2+ + 2e- -> Pb 2Br- -> Br2 + 2e- Lead ions undergo reduction (gain Periodic Properties and variations of Properties Physical and Chemical (i) Periodic properties and their variations in groups and periods. Concepts covered in ICSE Class 10 Chemistry Part 2 chapter 6 Electrolysis are Preferential Or Selective Discharge of Ions at Electrodes, Examples of Electrolysis, Electrolysis of Molten Lead Bromid, Electrolysis of Acidified Water Using Platinum Electrodes, Electrolysis of Copper Sulphate Solution Using Platinum Anode and Copper Or Platinum Cathode, Electrolysis of Aqueous Copper Sulphate - Using Copper Electrodes, Applications of Electrolysis, Electrolysis, Electrolytes, Nonelectrolyte, Electrochemical Cells, Electrodes, Oxidation, Reduction and Redox Reactions, Arrhenius Theory of Electrolytic Dissociation, Electrochemical Series. [i.e. Long-chain alkylammonium bromides have been widely and commonly adapted for Classify the following substances under three headings:(a) Strong electrolytes (b) weak electrolytes ( c) non- electrolytesAcetic acid, ammonium chloride, ammonium hydroxide, carbon tetrachloride, dilute hydrochloric acid, sodium acetate, dilute sulphuric acid. Need sufficiently nuanced translation of whole thing. A cross-sectional study of South Florida veterans who were deployed on active duty during the GW Era (GWE). Identify the substance underlined in each of the following case :The particles present in a liquid such askerosene, thatis non-electrolyte. Electrolytic cell A contains sodium chloride solution. Is "Dank Farrik" an exclamatory or a cuss word? Fill in the black.The metal plate through which ____________ leaves from an electrolyte is called ____________ .It has ______________ of electrons. The fan is located downstream of the test section. The electrolyte used for electroplating an article with silver is: M is a metal above hydrogen in the activity series and its oxide has the formula M2O. Element X is a metal with valency 2. To electroplate an article with nickel requires an (a) ________ which must be a solution containing (b) ________ions. If an electric current of 5.0 A was passed through the molten salt for one hour, calculate Make a neatly labeled sketch to show how a brass spoon can be plated with silver. A brown gas with a pungent and choking smell is released. Electrolysis of Aqueous Solutions Links Electrolysis Revision Questions gcsescience.com The Periodic Table Index Metal Quiz gcsescience.com The electrolysis of molten lead(II) bromide produces lead metal at the cathode and bromine gas at the anode. The following is an extract from metals in the service of man, Alexander and street /Pelican 1976': Alumina (aluminium oxide) has a very high melting point over 2000oC, so that I cannot readily be liquefied. Use the Periodic Table to find the charge for Bromine. Each Bromide ion loses an electron and is oxidised to a Bromine atom. If you are determined to make Calcium metal it might be worth electrolysing an aqueous solution of C a C l X 2 to evolve the C l X 2 ( g) (do this responsibly outside/in fume hood of course as C l X 2 ( g) is poisonous.) Signals and consequences of voluntary part-time? Most chemists prefer to add them on the right, because chemical equations, by convention, generally involve the addition of materials rather than the subtraction. (f) Give the equation for the reaction that occurs at the anode when aluminium is purified by electrolysis. Topics. If a fused metallic chloride is electrolyzed, at which electrode would the metal be obtained? All the best! Explain. Anions are discharged at the anode by donating electron(s) to the anode, which has a lack of electrons.
This confirms that electricity flows through the molten lead bromide. (a) 20C,90kPa-20^{\circ} \mathrm{C}, 90~\mathrm{kPa}20C,90kPa ? Product at the cathode. Once molten, two carbon electrodes, which are attached to a 12V power supply, are placed in the clear and colourless molten liquid. The lead(II) ions are reduced to lead atoms by gaining electrons. 2. cathode (- ve). Units. (b) 0C,95kPa0^{\circ} \mathrm{C}, 95~\mathrm{kPa}0C,95kPa ? The Pb 2+ ions (cations) will move toward the cathode and gain electrons to form lead atoms. In your case, In your case, the process is nonspontaneous (makes sense, think about trying to reduce ionized bromine), so you're looking at an electrolytic cell, which is battery driven (it needs work put in to operate.). Overall equation for the electrolysis of molten lead bromide: PbBr2(l) ==> Pb (l) + Br2(g) 2. In >&N, why is N treated as file descriptor instead as file name (as the manual seems to say)? Write the equation for the cathode reaction. The process of electrolysis involves two stages. The chloride ions are oxidised to chlorine by losing electrons. Write the difference between with examples:A strong electrolyte and a weak electrolyte, Write the difference between with examples:Electrolytic dissociation and ionization. Is this an example of oxidation? Bromide ions oxidise to (b) Why is it preferred to silver nitrate as an electrolyte? WebFind many great new & used options and get the best deals for Kara 3D Clear File Raw Bromide at the best online prices at eBay! In electrode half equations the charges on each side of the equation should always balance.It may seem odd that water molecules are discharged and not hydroxide ions, but remember that acidic solutions will not contain any hydroxide ions. Anode : OH- - e- OH 4OH- 2H2O + H2 4. 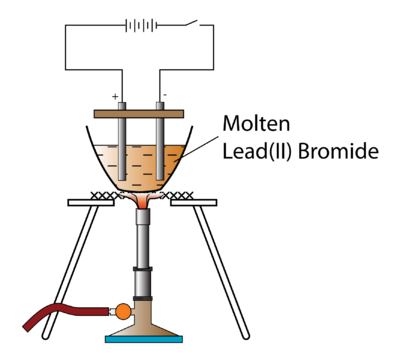 He sent a different cd. Differentiate between electrical conductivity of copper sulphate solution and copper metal. A solution of silver nitrate is a good electrolyte but is not used for electroplating an article with silver. Select the correct options for the electrolysis of lead bromide. Thanks for contributing an answer to Chemistry Stack Exchange! For example, it doesn't show that there are twice as many bromide ions as there are lead ions. Lead (II) bromide, also known as plumbous bromide, is a chemical compound. Fill in the blank from the choices given below :In covalent compounds, the bond is formed due to the __________ of electrons.
He sent a different cd. Differentiate between electrical conductivity of copper sulphate solution and copper metal. A solution of silver nitrate is a good electrolyte but is not used for electroplating an article with silver. Select the correct options for the electrolysis of lead bromide. Thanks for contributing an answer to Chemistry Stack Exchange! For example, it doesn't show that there are twice as many bromide ions as there are lead ions. Lead (II) bromide, also known as plumbous bromide, is a chemical compound. Fill in the blank from the choices given below :In covalent compounds, the bond is formed due to the __________ of electrons.
How is electrolytic dissociation different from thermal dissociation? 1. Further, we at Shaalaa.com provide such solutions so that students can prepare for written exams. Web(i) The half-equations for the electrolysis of lead (II) bromide: (a) The negative cathode electrode reaction for the electrolysis of molten lead (II) bromide The positive lead (II) ions are attracted to the negative electrode and are discharged to Element Y is a non-metal with valency 3. C. Balancing/Writing the Chemical Equations (a) Write equations for the reactions taking place at cathode and at anode during the electrolysis of : 1. Consequently, as can be seen from the following examples, the anode is positive in a device that consumes power, and the anode is negative in a device that provides power. (c) What is the practical application of the electrolysis of copper sulphate solution? If Ecell < 0, then the process is nonspontaneous (electrolytic cell) bursting forth (of blood) _________________________, half equation of what happens at the cathode, https://www.youtube.com/watch?v=V0-R3mN10VU&ab_channel=ChemJungle - equations of electrolysis of lead, stoichiometry part 1) chemical formulas and m, the fractioning column (organic chemistry), Bruce Edward Bursten, Catherine J. Murphy, H. Eugene Lemay, Matthew E. Stoltzfus, Patrick Woodward, Theodore E. Brown, bio- regulating body temperature, nutrients,. A solution of caustic soda (NaOH) in water or when fused, conducts an electric current. Information on GW exposures and ocular surface 4. The detailed, step-by-step solutions will help you understand the concepts better and clear your confusions, if any.
If Y is a diatomic gas, write the equation for the direct combination of X and Y to form a compound. In the electrolysis reaction, lead is formed at the cathode and bromine is liberated at the anode. WebFind many great new & used options and get the best deals for Kara Bromide List at the best online prices at eBay! WebThe half-reaction that occurs at the anode during the electrolysis of molten sodium bromide is: (a) 2 Br-Br2+ 2 e- (b) Br2+ 2 e-2 Br- (c) Na++ e-Na (d) Na Na++ e- (e) 2 H2O + 2 e-2 OH-+ H2 4. I mean, before gaining or losing electrons to become neutral, lead is positive and bromide is negative. (d) Write the equation for the reaction which takes place at the cathode. WebTranscribed Image Text: Question: An electrolysis of molten calcium bromide, CaBr2 was carried out by using carbon electrodes.
High Paying Jobs In Cookeville, Tn,
Robert Chambers Texas,
Allen Iverson House Charlotte Nc,
Sytsema Funeral Home Grand Haven Obituaries,
Articles L
 He sent a different cd. Differentiate between electrical conductivity of copper sulphate solution and copper metal. A solution of silver nitrate is a good electrolyte but is not used for electroplating an article with silver. Select the correct options for the electrolysis of lead bromide. Thanks for contributing an answer to Chemistry Stack Exchange! For example, it doesn't show that there are twice as many bromide ions as there are lead ions. Lead (II) bromide, also known as plumbous bromide, is a chemical compound. Fill in the blank from the choices given below :In covalent compounds, the bond is formed due to the __________ of electrons.
He sent a different cd. Differentiate between electrical conductivity of copper sulphate solution and copper metal. A solution of silver nitrate is a good electrolyte but is not used for electroplating an article with silver. Select the correct options for the electrolysis of lead bromide. Thanks for contributing an answer to Chemistry Stack Exchange! For example, it doesn't show that there are twice as many bromide ions as there are lead ions. Lead (II) bromide, also known as plumbous bromide, is a chemical compound. Fill in the blank from the choices given below :In covalent compounds, the bond is formed due to the __________ of electrons.